Change to Future Approvals
Coming Soon! – A change will be made to the approval process for Proposal Development documents. When proposals are submitted into route, the person submitting the proposal will no longer be able to approve for future approval requests. However, if someone is an approver at the Department and College levels, when approving at the Department level they will receive a message asking, “Would you like the College to have another chance to approve?” If answered “yes”, the proposal will need to be approved again at the College level. If answered “no”, the person’s approval at the Department level will also count toward the College level. We plan to implement this change within the next few weeks.
New Human Subjects Exemptions
On January 21, 2019 amendments to the Federal Policy for the Protection of Human Subjects (Common Rule) will go into effect. With this change two additional exemptions are being added, exemptions 7 and 8, which are now included in the Exemption # list in KC. The letter in front of the exemption number has been removed to accommodate differences between agencies. For assistance with determining an exemption under the Revised Common Rule, please see the following resource provided by NIH: https://grants.nih.gov/sites/default/files/exemption_infographic_v6_508c-1-7-19rev.pdf. 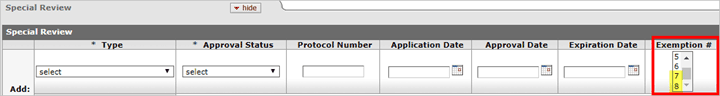
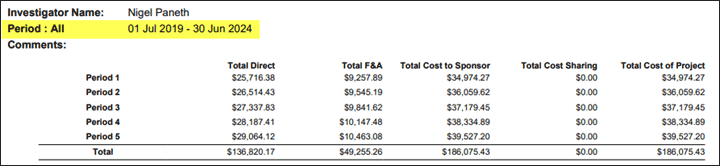
Budget Period Dates Added to Report 1, 2 and 4
In budget reports 1 and 2, dates have been added to the top of each budget period. 
In budget reports 1, 2 and 4, the project period has been added to the budget summary.