Some application packages pull in the complete list of forms from the Find an Opportunity lookup. Other application packages have mandatory or optional forms that do not automatically pull in, and will need to be attached as User Attached Forms (UAF). You will not be able to route a proposal until mandatory User Attached Form(s) are attached. Instructions on how to attach a User Attached Form are provided below.
To identify when a User Attached Form is needed, look at the Description column. If the description is Unavailable a User Attached Form must be used to submit the form.
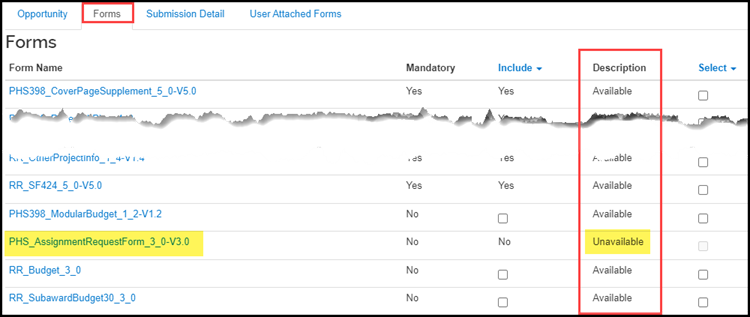
If the form is mandatory for submission, the Mandatory column will say Yes.
To attach a User Attached Form take the following steps:
- From the Forms tab, click the hyperlink for the Form Name. This will open a PDF version of the fillable form.
- Complete the fillable form and save it to a location where you can easily find it.
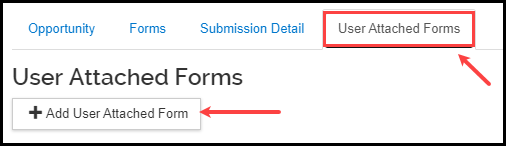
- Click the User Attached Forms tab, click the +Add User Attached Form button.
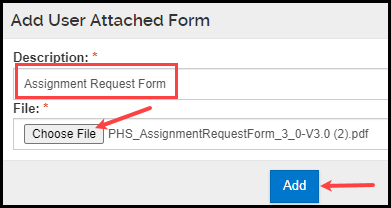
- Within the pop-up window that appears, add a Description for your attachment and select the appropriate file. Click Add.
- For optional User Attached Forms, the Forms tab will change to a checked box in the Include column and the Description column will say User Attached Form.

- For mandatory User Attached Forms, the Forms tab will change to display Yes in the Include column and the Description column will say User Attached Form.

To replace a user attached form:
- Click the Actions button under the Actions column in the User Attached Forms tab.
- Select the Delete option.

- A popup window will appear asking if you are sure you want to delete. Click OK.
- Re-add the revised User Attached Form following the above instructions for attaching a UAF.
Notes on User Attached Forms:
- Some Grants.gov opportunities utilize forms that have expired. This is something that is set by Grants.gov and not Kuali. The proposal must utilize the version of the form that is pulled in from Grants.gov, even if the form has expired.
- Subaward budget forms are attached in the Budget module, under the Subawards option.
- If a User Attached Form includes additional attachments that need to be replaced, you must delete the UAF, fix the attachments, and re-add the UAF. This can be done while the proposal development document is in route, or any time prior to OSP’s approval.